Events
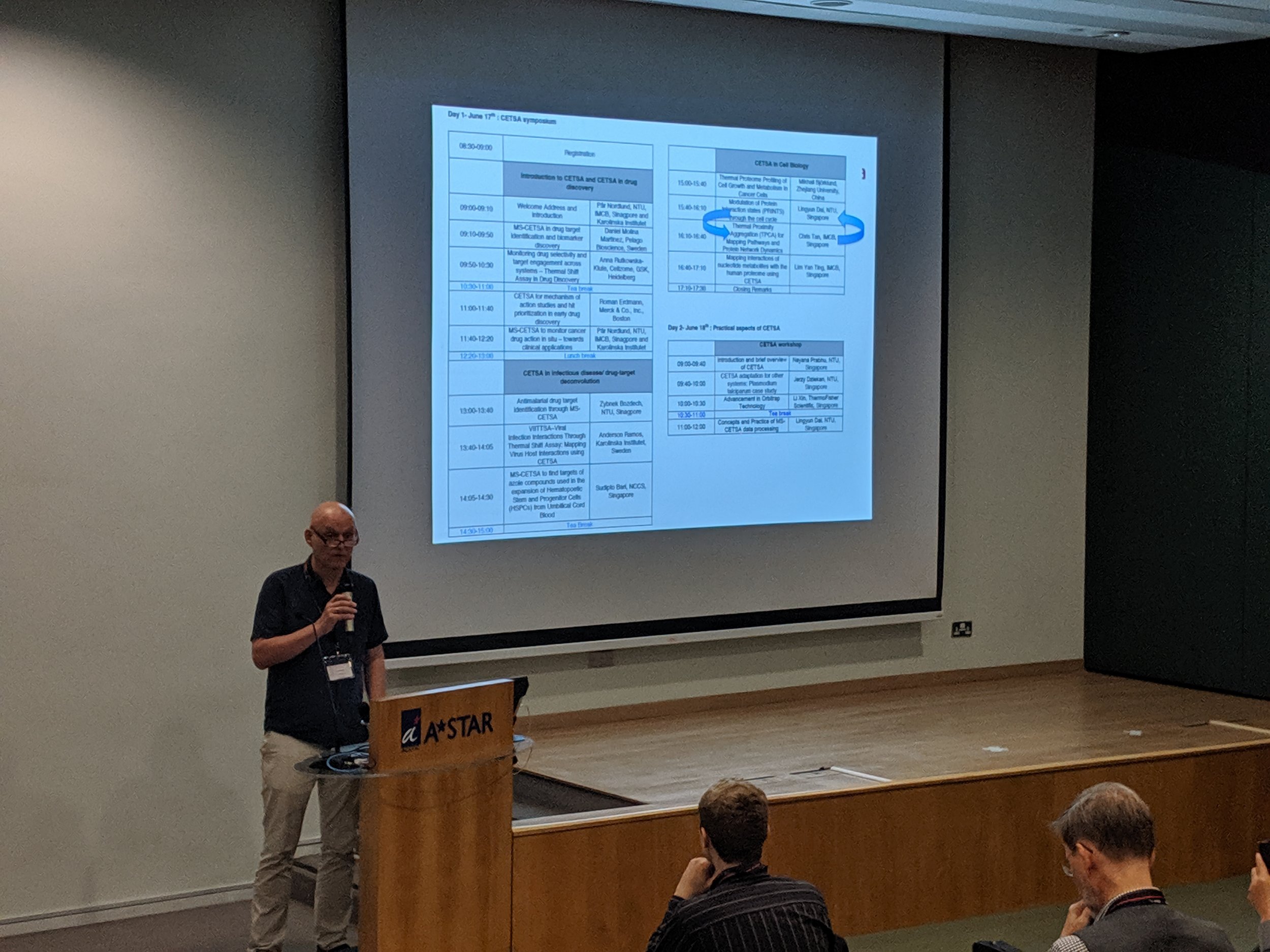
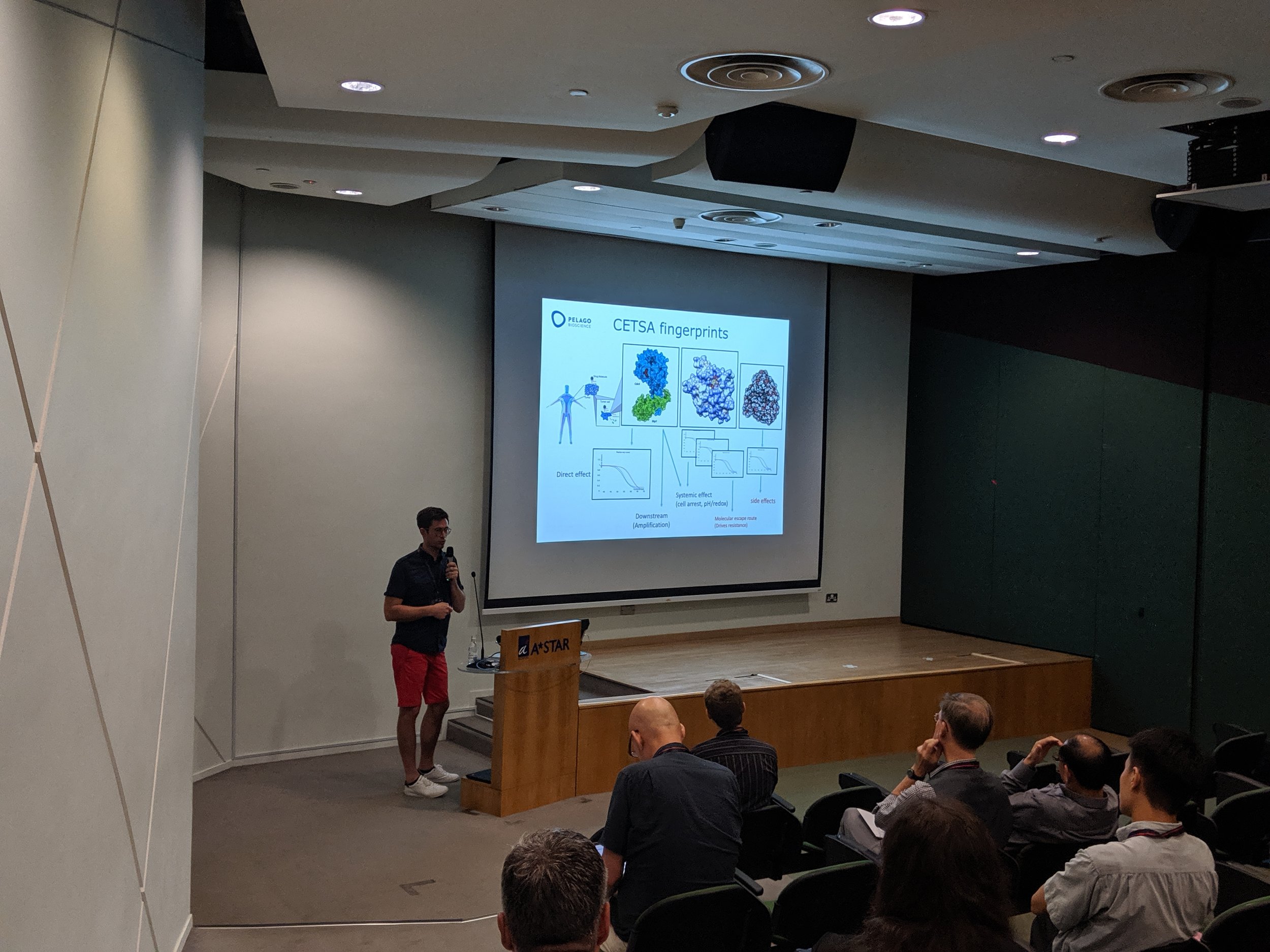
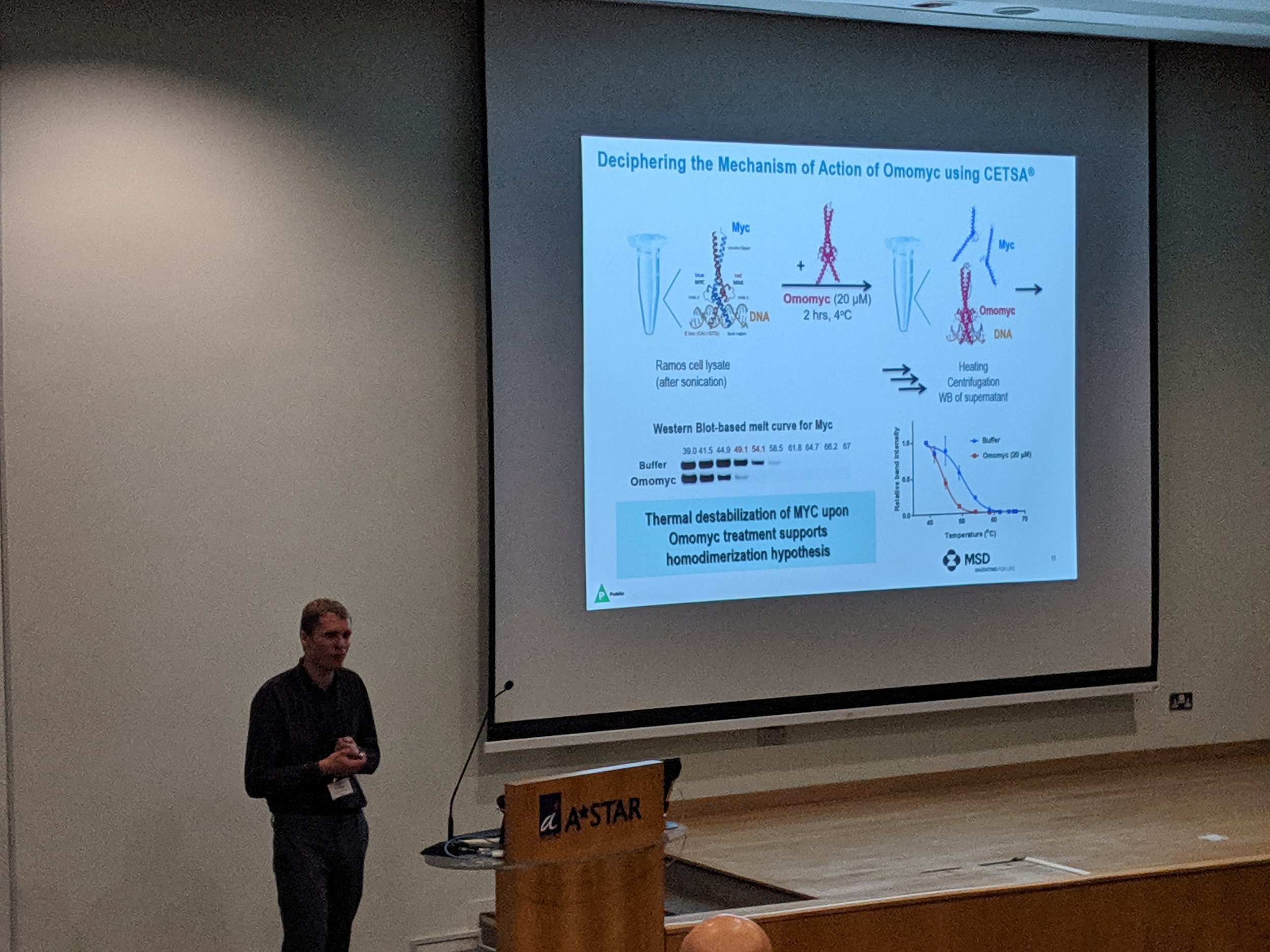
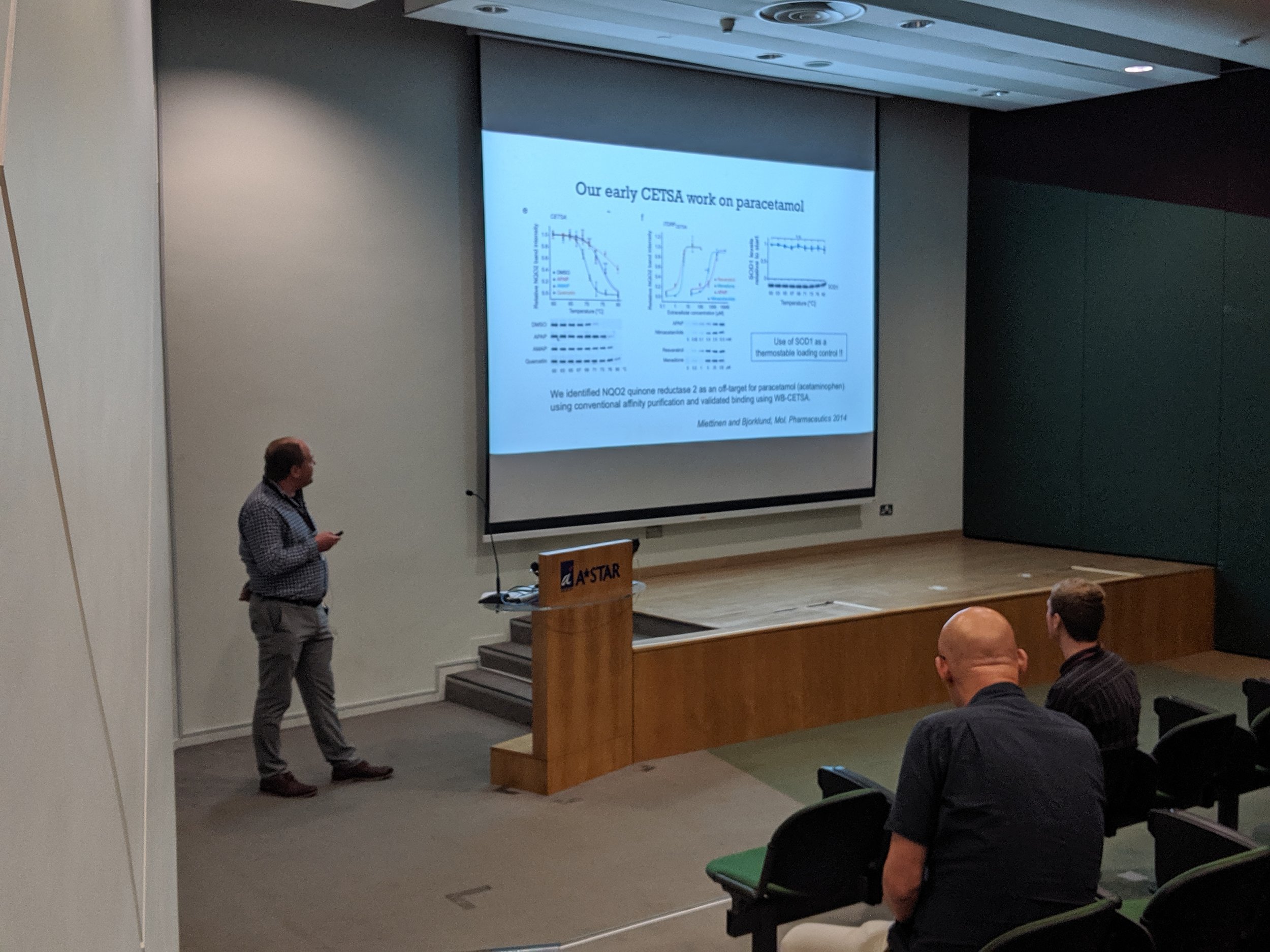
The first Singapore CETSA meeting was a success, thanks to a wide participation. See snapshots from the two day meeting below.





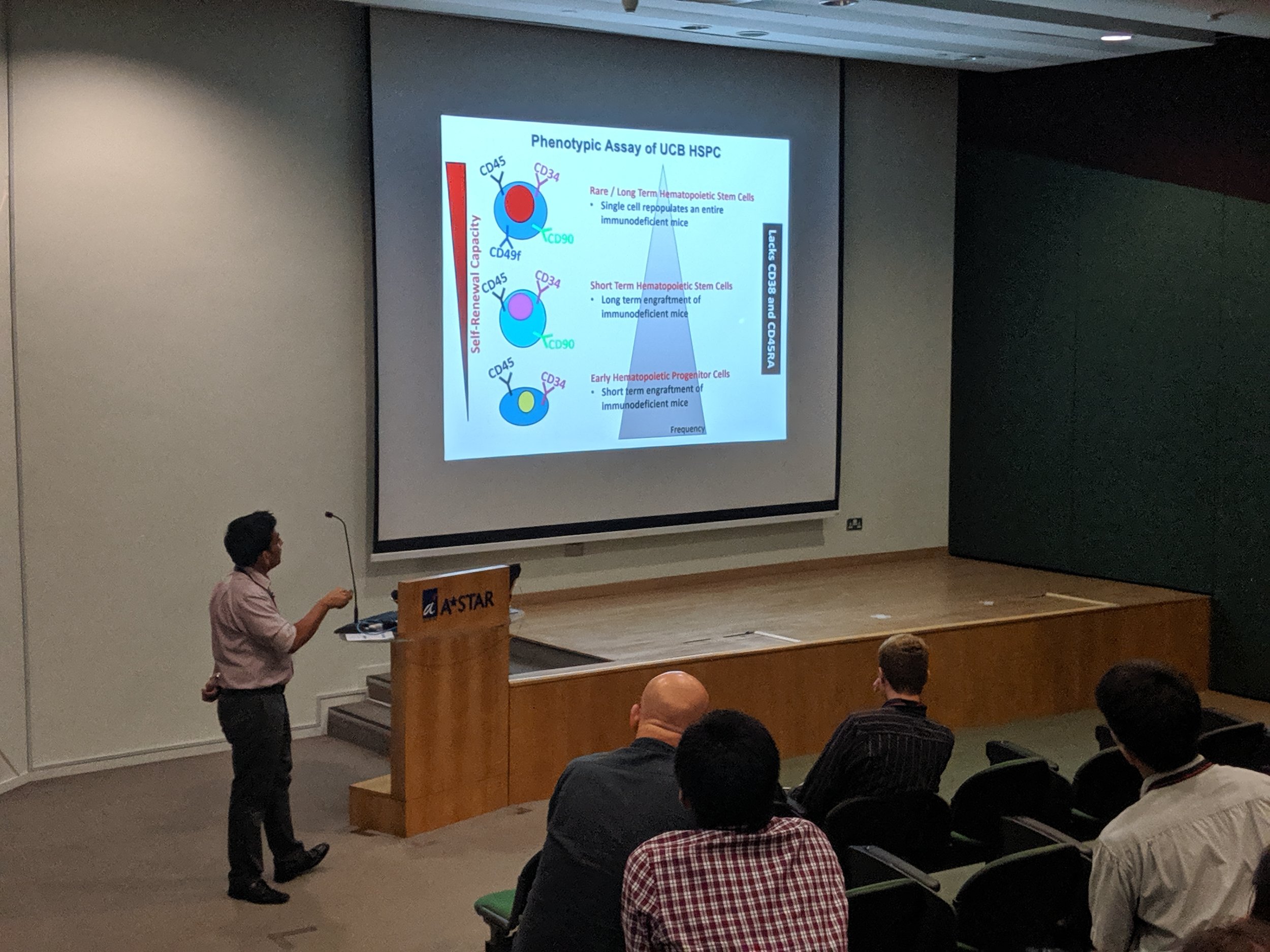
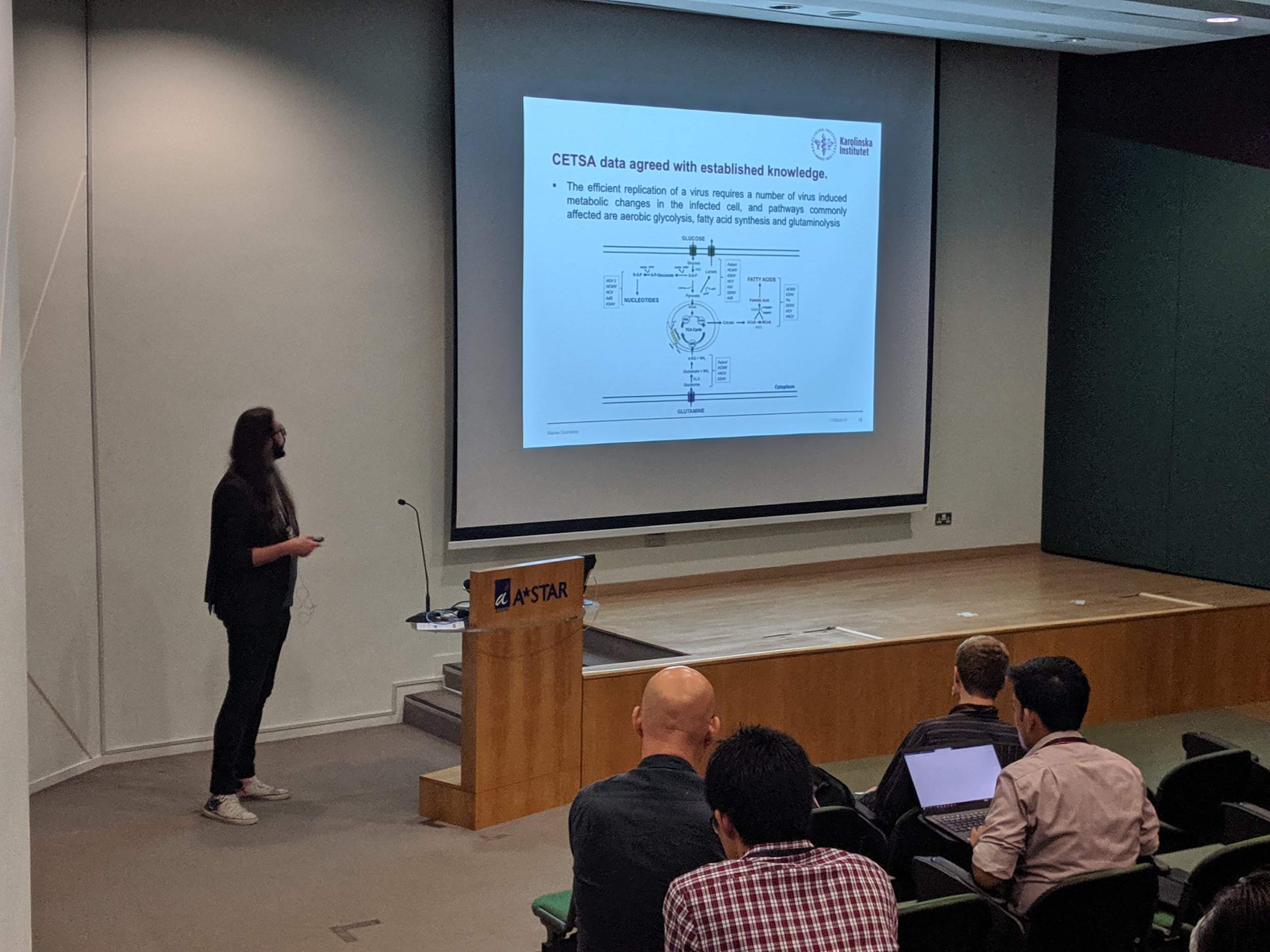



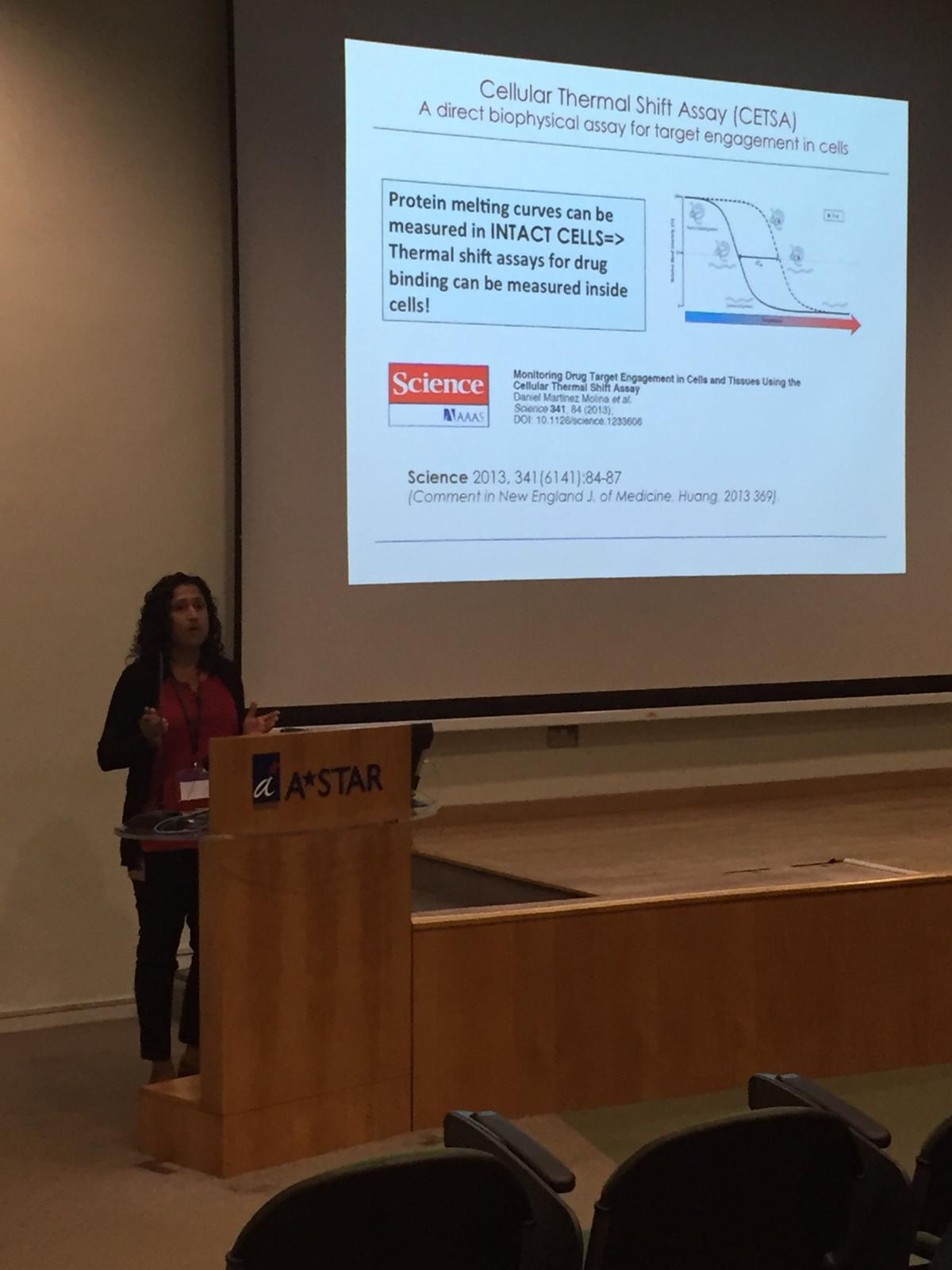



Our most recent publications
Liang YY, Bacanu S, Sreekumar L, Ramos AD, Dai L, Michaelis M, Cinatl J, Seiki T, Cao Y, Coffill CR, Lane DP, Prabhu N, Nordlund P (2021). CETSA interaction proteomics define specific RNA-modification pathways as key components of fluorouracil-based cancer drug toxicity. Cell Chem Biol, 2021 S2451-9456(21)00306-8.
Sun J, Prabhu N, Tang J, Yang F, Jia L , Xiao K, Tam WL, Nordlund P, Dai L (2021). Recent advances in proteome-wide free target deconvolution for bioactive small molecules. Med Res Rev, 21788 (Online ahead of print)
Prabhu N, Dai L, and Nordlund P (2020). CETSA in integrated proteomics studies of cellular processes Curr Opi Chem Biol, Feb;54:54-62
Dziekan JM, Wirjanata G, Dai L, Go KD, Yu H, Lim YT, Chen L, Wang LC, Puspita B, Prabhu N, Sobota RM, Nordlund P, Bozdech Z (2020). Cellular thermal shift assay for the identification of drug-target interactions in the Plasmodium falciparum proteome Nature Protocols, 15(6):1881-1921
Langebäck A, Bacanu S, Laursen H, Mout L, Seki T, Erkens-Schulze S, Ramos AD, Berggren A, Cao Y, Hartman J, van Weerden W, Bergh J, Nordlund P, Lööf S (2019). CETSA based target engagement of taxanes as biomarkers for efficacy and resistance. Sci Rep 2019 12;9(1):19384
Dai L, Prabhu N, Liang YY, Bacanu S, Ramos A, Nordlund P. (2019) Horizontal Cell Biology: Monitoring global changes of protein interaction states in cells with proteome-wide CETSA, Annual Reviews of Biochemistry, 88:383-408
Other CETSA publications
2018
Becher I, Andrés-Pons A, Romanov N, Stein F, et al. Pervasive Protein Thermal Stability Variation during the Cell Cycle. (2018) Cell. DOI: 10.1016/j.cell.2018.03.053
Brent D. G. Page, Nicholas C. K. Valerie, Roni H.G. Wright, Olov Wallner, et al. Targeted NUDT5 inhibitors block hormone signaling in breast cancer cells. (2018) Nature Communications. DOI: 10.1038/s41467-017-02293-7
Axelsson H, Almqvist H, Otrocka M, Vallin M, et al. In Situ Target Engagement Studies in Adherent Cells. (2018) ACS Chem. Biol. DOI: 10.1021/acschembio.7b01079
Shaw J, Leveridge M, Norling C, Karén J, Molina DM, O’Neill D, Dowling JE, Davey P, Cowan S, Dabrowski M, Main M, Gianni D. Determining direct binders of the Androgen Receptor using a high-throughput Cellular Thermal Shift Assay. (2018) Nature, Scientific REPORTS. DOI: 10.1038/s41598-017-18650-x
Shaw J, Dale I, Hemsley P, Leach L, et al. Positioning High-Throughput CETSA in Early Drug Discovery through Screening against B-Raf and PARP1. (2018) Sage Journals. DOI: 10.1177/2472555218813332
2017
Kettle JG, Alwan H, Bista M, Breed J, Davies NL, et al. Potent and Selective Inhibitors of MTH1 Probe Its Role in Cancer Cell Survival. (2017) Journal of Medicinal Chemistry DOI: 10.1021/acs.jmedchem.5b01760
Mayumi Kitagawa, Pei-Ju Liao, Kyung Hee Lee, Jasmine Wong, See Cheng Shang, et al. Dual blockade of the lipid kinase PIP4Ks and mitotic pathways leads to cancer-selective lethality. (2017) Nature Communications. DOI: 10.1038/s41467-017-02287-5
Schuetze KB, Stratton MS, Blakeslee WW, Wempe MF, Wagner FF, et al. Overlapping and Divergent Actions of Structurally Distinct HDAC Inhibitors in Cardiac Fibroblasts. (2017) J. Pharmacol. Exp. Ther. DOI: https://doi.org/10.1124/jpet.116.237701
Tsuyoshi Ishii, Takuro Okai, Misa Iwatani-Yoshihara, et al. CETSA quantitatively verifies in vivo target engagement of novel RIPK1 inhibitors in various biospecimens. (2017) Nature Scientific Reports. DOI: 10.1038/s41598-017-12513-1
2016
Almqvist H, Axelssson H, Rozbeh J, Dan C, Mateus A, Haraldsson M, Larsson A, Martinez Molina D, Artursson P, Lundbäck T, Nordlund P. CETSA screening identifies known and novel thymidylate synthase inhibitors and slow intracellular activation of 5-fluorouracil. (2016) Nature Communications 7:11040
Exell JC, Thompson MJ, Finger LD, Shaw SJ, Debreczeni J, et al. Cellularly active N-hydroxyurea FEN1 inhibitors block substrate entry to the active site. (2016) Nature Chem. Biol. DOI:10.1038/nchembio.2148
Martinez Molina D, Nordlund P. The Cellular Thermal Shift Assay: A Novel Biophysical Assay for In Situ Drug Target Engagement and Mechanistic Biomarker Studies. (2016) Annu. Rev. Pharmacol. Toxicol. 56:141-161
Thorsell A-G, Ekblad T, Karlberg T, Löw M, Pinto A F, Tresaugues L, Moche M, Cohen M S and Shüler H. Structural basis for potency and promiscuity in poly(ADP-ribose) polymerase (PARP) and tankyrase inhibitors. (2016) J. Med. Chem. DOI: 10.1021/acs.jmedchem.6b00990
Xu H, Gopalsamy A, Hett E, Salter S, Aulabaugh A, Kyne R, Pierce B, Jones LH. Cellular Thermal Shift and Clickable Chemical Probe Assays for the Determination of Drug-Target Engagement in Live Cells. (2016) Org. Biomol. Chem. DOI: 10.1039/C6OB01078D
2015
Chan-Penebre E, Kuplast KG, Majer CR, Boriack-Sjodin PA, Wigle TJ, et al. A selective inhibitor of PRMT5 with in vivo and in vitro potency in MCL models. (2015) Nat. Chem. Biol. 11:432–3
Franken H, Mathieson T, Childs D, Sweetman GMA, Werner T, et al. Thermal proteome profiling for unbiased identification of direct and indirect drug targets using multiplexed quantitative mass spectrometry. (2015) Nature Protocols 10:1567-1593
Malik R, Khan AP, Asangani IA, Cieślik M, Prensner JR, et al. Targeting the MLL complex in castration-resistant prostate cancer. (2015) Nat. Med. 21:344–52
Qin JJ, Wang W, Voruganti S, Wang H, Zhang WD, Zhang R. Identification of a new class of natural product MDM2 inhibitor: in vitro and in vivo anti-breast cancer activities and target validation. (2015) Oncotarget 6:2623–40
Reinhard FBM, Eberhard D, Werner T, Franken H, Childs D, et al. Thermal proteome profiling monitors ligand interactions with cellular membrane proteins. (2015) Nature Methods 12:1129-1131
Sidrauski C, Tsai JC, Kampmann M, Hearn BR, Vedantham P, et.al. Pharmacological dimerization and activation of the exchange factor eIF2B antagonizes the integrated stress response. (2015) eLife 4:e07314
Tan BX, Brown CJ, Ferrer FJ, Yuen TY, Quah ST, et al. Assessing the efficacy of Mdm2/Mdm4- inhibiting stapled peptides using cellular thermal shift assays. (2015) Sci. Rep. 5:12116
2014
Bai L, Chen J, McEachern D, Liu L, Zhou H, et al. BM-1197: a novel and specific Bcl-2/Bcl-xL inhibitor inducing complete and long-lasting tumor regression in vivo. (2014) PLOS ONE 9:e99404
Bradley WD, Arora S, Busby J, Balasubramanian S, Gehling VS, et al. EZH2 inhibitor efficacy in non-Hodgkin’s lymphoma does not require suppression of H3K27 monomethylation. (2014) Chem. Biol. 21:1463–75
Huber KVM et al. Stereospecific targeting of MTH1 by (S)-crizotinib as an anticancer strategy. (2014) Nature 508(7495na):222–227
Jafari R, Almqvist H, Axelsson H, Ignatushchenko M, Lundbäck T, Nordlund P, Martinez Molina D. The cellular thermal shift assay for evaluating drug target interactions in cells. (2014) Nature Protocols 9(9):2100-2122
Khoo KH, Verma CS and Lane DP. Drugging the p53 pathway: understanding the route to clinical efficacy. (2014) Nature Reviews Drug Discovery 13(3):217-236
Miettinen TP, Björklund M. NQO2 is a reactive oxygen species generating off-target for ac- etaminophen. (2014) Mol. Pharm. 11:4395–404
Sackton KL, Dimova N, Zeng X, Tian W, Zhang M, et al. Synergistic blockade of mitotic exit by two chemical inhibitors of the APC/C. (2014) Nature 514:646–49
Savitski MM, Reinhard FBM, Franken H, Werner T, Savitski MF, Eberhard D, Martinez Molina D, et al. Tracking cancer drugs in living cells by thermal profiling of the proteome. (2014) Science 346. DOI: 10.1126/science.1255784
